


Sources of ElectricityAlthough electricity is a naturally occurring phenomena, in its natural forms, (Lightening, Static Electricity, etc) it has been of little use to mankind. We have, however, invented ways to generate electricity. Electricity can be generated by Chemical, Magnetic, or Transducer methods. Chemical :I learned 40 some years ago, that the earliest records of Electronics finds the early Egyptians around the time of Moses, having discovered how to make a crude battery using lemons (which have citric acid) and two dissimilar metals. Interesting story, albeit not provable. In fact, the earliest "feasable" battery might have been a battery-like artifact was unearthed at Khuyut Rabbou'a near Baghdad dubbed the "Baghdad Battery". However, it likely was not a battery at all, but rather a storage container for important documents. In truth, the first true battery, the "Voltaic Pile" was not created until around the year 1800 by Alessandro Volta - the man we name the unit of measurement of electrical pressure (the Volt) after. In any case, all batteries produced using two dissimilar conductive materials into an acid ELECTROLYTE . Modern batteries are a little more complex than a couple of lemons though: 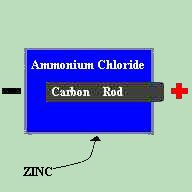 Perhaps the most common battery in use today is the dry cell, which can be
found in nearly every flashlight!
Perhaps the most common battery in use today is the dry cell, which can be
found in nearly every flashlight!
The dry cell contains a carbon rod, which acts as the positive terminal, surrounded by a core consisting of manganese dioxide, zinc chloride, glycerin, carbon particles, and sawdust. Around this core is a chemical paste made up of an ammonium chloride solution in starch. A zinc can is then used as the container for the cell and also acts as the negative terminal. The carbon rod reacts with the zinc casing via the pasty electrolyte. This creates a 1.5 Volt potential difference between the positive and negative terminals of the cell. It is not the electrolyte, but the electrodes themselves (the zinc and carbon) which determine the voltage of the cell. Therefore, no matter which electrolyte is chosen to be placed between the zinc and the carbon, the cell will still produce 1.5 Volts. So how do we come up with a 9 Volt battery? Simple. By placing cells in series, we can add the Voltages together. Two 1.5 Volt cells in series would produce a 3 Volt output. 3 would product 4.5 Volts. 6 would produce 9 Volts. When we place cells in series, we say that it is a BATTERY of cells. A close cousin to the lemon batteries is the modern LEAD-ACID type battery. Commonly used as automotive type batteries, the negative electrode is made up of pure lead and the positive electrode is a lead-peroxide combination. The electrolyte nominally used is a diluted sulfuric acid. A single lead-acid cell produces 2.1 Volts, but these batteries are commonly produced in either 6.3 or 12.6 Volts. |
| (On The Following Indicator... PURPLE will indicate your current location) | ||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
| 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 |
| 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 |
| Otherwise - please click to visit an advertiser so they know you saw their ad! |
