


|
*Note: This portion of the course is intended to give a more thorough understanding of the chemistry and solid state physics of semiconductor devices. It is not intended for students lower than high school students. Its key purpose is to present cognitive thought paterns and alternate ideas concerning the very theory of electron flow. In order to understand how semiconductors work, we have to go back to the origins of their discovery. Chemistry! Remember in the beginning of our course, we went over the periodic table of the elements? We did this because electricity is based upon the movement of electrons, which are microscopic particles contained within the atoms of the elements themselves. What we did not discuss in detail is exactly how an atom is put together. An atom is made up like a little Solar System. Just like our Sun has planets revolving around it in orbit, on a much smaller, microscopic scale, atoms have a center, with tiny little "planets" orbiting around it. The center, you already know is a nucleus, and the "planets" are electrons. The electrons orbit around the nucleus in "bands", and only a certain amount of electrons can fit in each band. Of course - this is a very ordered device, otherwise all the particles in it would spin around randomly, and left completely undisturbed would occasionally crash into each other causing chemical and physical instabilities, and randomly forming unpredictable reactions. Without getting into Hund's rule and energy levels, the bands basically fill up from the innermost to the outermost bands. In the innermost band, 2 electrons are allowed. The second band from the inside will accept up to 8 electrons. The third band can have up to 18 electrons and so on. The atoms, much like our solar system, are held together by gravitational pulls, centrifugal forces, and even some forces we don't yet understand. What we do know for certain, is that an ordinary atom is electrically balanced, because the electrical charge of one proton is exactly equal to the charge of one electron, and all ( normal ) atoms have the exact same amount of protons and electrons. Since any balanced atom has the same amount of protons and electrons, the two charges nullify and the electrical charge of the atom remains neutral. If for some strange reason, an atom should lose or gain an electron, and become electrically unbalanced, it is considered a positive or negative ion, depending on whether it lost an electron ( becoming positive ), or gained one ( becoming negative ). 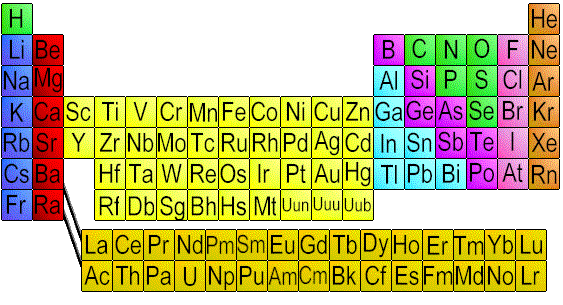
Each chemical element in the periodic chart is laid out in rows ordering from left to right according to how many protons it has. For instance Hydrogen (H) has one proton. Helium (He) has 2 protons. Lithium (Li) has 3, Beryllium (Be) has 4, Boron (B) has 5 and so on. The outermost ring of any atom is called the valence ring. 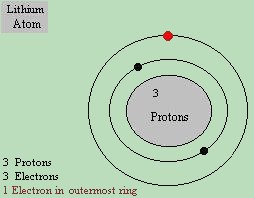 In each normal atom, there are exactly the same amount of electrons as protons, so if Helium has 2 protons, it also has 2 electrons. In this case, there are only 2 electrons allowed in the outermost ring, so it's valance ring is full. Lithium, however, with 3 electrons, can only have 2 electrons in the first ring, so the third electron forms the beginning of a second orbital ring.
In each normal atom, there are exactly the same amount of electrons as protons, so if Helium has 2 protons, it also has 2 electrons. In this case, there are only 2 electrons allowed in the outermost ring, so it's valance ring is full. Lithium, however, with 3 electrons, can only have 2 electrons in the first ring, so the third electron forms the beginning of a second orbital ring. This second ring can have up to 8 elelctrons before it is full. Therefore, Neon, with 10 electrons has both it's first and second ring full. All elements that have all their rings completely full are listed along the right side of the periodic table of elements, and are called "inert" . They are inert, because they do not react to other chemicals easily, and this is because their outer valance rings are full. Hydrogen, however has 1 electron orbiting about the nucleus to electrically balance it's 1 proton. Hydrogen is considered an "active" or element, because its atoms combine quickly and easily with many different elements to form molecules. 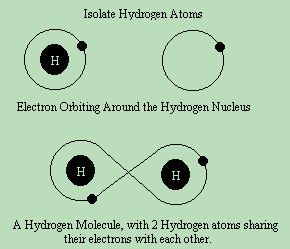 Atoms are normally found as part of a larger structure, called a molecule. If an atom were floating about in free space, it's electrons would be orbiting around it. But when combined with other atoms, it forms a molecule and sometimes shares it's electrons back and forth. This is possible, because by applying energy, you can knock an electron out of it's orbit. Much like one pool table ball will knock another one into the pocket.
We know that like charges repel. So if, for instance, in atom number 1, one electron comes too close to another one, it can knock it out of it's orbit. However, in the process, atom number 1 becomes a positive ion. Being positive, it attracts the electron from atom number 2, which freely gives it up. In this manner, they share the atoms back and forth, remaining 1 electron per atom at all times.
Atoms are normally found as part of a larger structure, called a molecule. If an atom were floating about in free space, it's electrons would be orbiting around it. But when combined with other atoms, it forms a molecule and sometimes shares it's electrons back and forth. This is possible, because by applying energy, you can knock an electron out of it's orbit. Much like one pool table ball will knock another one into the pocket.
We know that like charges repel. So if, for instance, in atom number 1, one electron comes too close to another one, it can knock it out of it's orbit. However, in the process, atom number 1 becomes a positive ion. Being positive, it attracts the electron from atom number 2, which freely gives it up. In this manner, they share the atoms back and forth, remaining 1 electron per atom at all times.The main point here, is that the chemical behavior of any particular element is determined by the number of electrons contained within its outermost ring. When the outer ring is filled, the atom does not have a desire for additional electrons, and it does not tend to go into a chemical combination with other atoms. Otherwise, if the outermost ring is not filled, the atom seeks other atoms with which it may lend to or borrow electrons from in order to complete its outermost shell. When two or more atoms are sharing someof the same electrons such that both atoms are satisfied, the two atoms form a molecule, and are said to be bonding.  Inter-activity of a given element is directly related to how well or poorly the element conducts electricity. If the outermost shell is full, it has no desire to share or borrow electrons, and remains an insulator. However, there are some elements which have one or more "extra" electrons way out on their outer ring, which will freely share this electron with others. These elements are conductors.
Inter-activity of a given element is directly related to how well or poorly the element conducts electricity. If the outermost shell is full, it has no desire to share or borrow electrons, and remains an insulator. However, there are some elements which have one or more "extra" electrons way out on their outer ring, which will freely share this electron with others. These elements are conductors. In an earlier section, we discussed that electrons appear to flow through Copper because they push the outermost electron away from the atom and into the next atom. We stated that this is why Copper conducts electricity. There are other metals that conduct electricity as well. There is a reason for this. If you look closely at the periodic table, you will find that Copper ( Cu ) is on the 4th row, and is the 11th chemical from the left. If you look directly below it, you find Silver ( Ag ), and directly below that is Gold ( Au ). Note that all three of these elements are soft metals, all can be shined to a high lustre, all are highly malable and all conduct electricity very well! In other words, they all have similar "properties". This is because they are from a similar "family". Chemicals in the periodic table are grouped by families, which are defined by their atomic structure (how many electrons in each ring), which also dictates that they have similar properties. 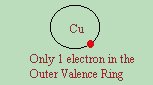 In the case of Copper, Silver, and Gold. All three have only a single electron in their outermost, or VALENCE ring.
In the case of Copper, Silver, and Gold. All three have only a single electron in their outermost, or VALENCE ring. While atoms want to remain electrically neutral, they by their nature, attempt to keep a certain number of electrons in each ring. Most importantly, they seek to keep their valance ring full. If it lacks 1 or 2 electrons from being full, it will try to borrow them from neighboring atoms. If, on the other hand, they only have 1 or 2 electrons in their outer ring, they try to lend them out so it appears that the ring below it is full, with no "extras" floating about. In the case of Copper, Silver, and Gold, the outer ring "appears" to have one electron too many, which sits way out on a far orbit. So the atom trys to give away this "extra" electron to "fill out" its valance ring. This electron lends itself to other atoms easily, and because of it's "distance" from the nucleus, can be easily knocked out of orbit to a neighboring atom. This electron in the outer ring is sometimes called a "free electron", because of it's tendency to lend itself out to other atoms. We know that electrons move very slowly through wire - although the PERCEPTION is that they move very quickly. Let me give you a couple of mental immages to think this through. You flip on a light switch - and the light bulb turns on instantly. Electricity must move at the speed of light right? Wull, think again. The wire that connects the switch to the bulb is made of (typically) copper. Simply for sake of this discussion - let's assume that our copper wire is exactly one copper atom in thickness. Copper has 1 electron in its outer ring that wants to be free. If we force a free electron into the wire (by turning on the switch) it doesn't instantly appear at the other end of the wire - instead, it pushes on the copper with force (electromotive force/ aka voltage). This pushes one of the copper's electrons out of place, and the free electron now takes the place of the copper's electron. That copper's electron has to go somewhere, so it pushes into the next copper atom's electron out of place, etc until finally, and the far end of the wire - a copper electron falls out of the wire, into the light bulb, and changes form from electricity to light and/or heat. Because this is very messy, and a bit abstract to understand, let me give 2 more simple examples. First, is the copper tube with marbles. We fill a 3 foot long copper tube with all white marbles, until all the marbles are touching each other, and it can contain no more marbles. Now we shove a black marble in the end of the pipe (representing the newly introduced electron). Because we pushed the black marble into the pipe, and the pipe is full of marbles, a marble has to fall out the other end. However - the marble that falls out is NOT the black marble we shoved in. It is a white marble. If we shove another black marble into the pipe, another white marble falls out and so forth. We have to shove a LOT of black marbles in before the FIRST black marble we shoved in falls out. This vaguely represents the time it takes for one electron to make it from one end of the wire to another - but wait - it is worse than that. Let's say your city is out of water, and you have to stand in line to get a gallon of water. If in your life you have been too fortunate to fathom that - simply trust me, it happens, I've seen it. I've also stood in line for gasoline, as well as movie theatre tickets. The principle is the same... So you stand in line you have to wait in line to get your water. You would love to move to the front of the line, but unless you FORCE your way up there, or find some shorter path - you simply have to wait. The first guy in line gets his gallon, and moves on out of the line, which leaves a hole in the line. The second person now can move up into the hole where the first person stood, leaving a hole where he used to be. The third guy moves up into the second guy's hole, the forth guy moves up into the 3rd guy's hole, and finally, when the person directly in front of you moves up - you can take up the empty space where he used to be. Interesting thing here - for every one person that eventually moved up to the front of the line, a hole eventually made its way to the back of the line. This is true in electronics too. Electricity does NOT move simply from positive to negative as in the Franklin theory, nor the other way around as in the Edison theory. The fact is, that as Electrons are moving from the back of the line to the front, HOLES are moving from the front to the back at the exact same rate and speed. No energy is ever lost or gained. For every action, there is an equal, but opposite reaction. Energy constantly seeks equilibrium. For every + that moves, there must also be a - that moves. To say then, that electricity only moves one way in a solid state diode is a misnomer. In reality - an electron (a negative charge) can not flow through a semiconductor, without a hole (a positive charge) moving through the same semiconductor at the exact same rate and speed, but in the opposite direction. It simply appears to do so when observing our limited test equipment. Having reviewed all this, we are now in a position to look at semiconductors. Semiconductors are also from similar atomic families, and have similar properties. Unlike conductors, they do not have only a single atom in their outer ring, but rather have 4. They are also very common elements, richly located throughout the world.  Silicon, for instance is a very common element -- for example, it is the main element that is found in quartz and sand. In the periodic table, Silicon ( Si ) is next to aluminum ( Al ), which is a highly conductive metal, below Carbon ( C ) and above Germanium ( Ge ), which are semi-conductors. Metals are usually good conductors of electricity because of the single "free" electron in the outer ring, which can easily move between atoms. In a semiconductive element, however, all of the outer ( valance ) electrons are interacting with other atoms in perfect covalent bonds, so they just can't move around that easily. Because of that, a pure semiconductive crystal is almost an insulator. The miracle of semiconductors happens because you can change the characteristics of a semiconductive element by "DOPING" it. Doping is the term used for when you mix a small amount of an impurity into the crystal lattice. There are two types of impurities that we can add in:
Now this is a little difficult to explain without going deep into how valance rings, atomic numbers, chemistry, and solid state physics works, but I'm gonna give it a try: In the outer ring of a Silicon atom, for instance, there are 4 electrons. For what we'll call emotional reasons ( it's suffering from a dependency complex ), it wants 8. So if a spare one or two comes along, it is happy to accept them. But what happens if 5 extra electrons come along? If we look to the right of Silicon on the chart, we find Phosphoros ( P ). Phosphorus has a total of 5 electrons in the outer ring. So if we combine 1 Phosphorus atom with 4 Silicon atoms, we have a molecule that has an "extra" electron just hanging around. It's ready to go on the move. In short... it's a "free" electron. We have turned the silicon molecule into a conductor. Because the molecule has an "extra" electron, it is Negative in nature, and wants to give it's extra electron away. Thus we call it an N-Type Doping. Because of the way elemental families work, we don't have to use Phosphorus for this. We could just as easily use Arsenic ( As ) or another element from that columb and mix it with Germanium ( Ge ), or aother element from the semiconductor column.  Now let's look to the left side of Silicon on the chart. Here we have Boron ( B ), Gallium ( Ga ), and other elements. These elements have ( you guessed it ) 3 electrons in the valance ring. If we Dope the Silicon with Gallium or Boron, we wind up with a molecule that has only 7 of the 8 electrons it wants ( 4 from the Silicon, 3 from the Gallium ). Because it wants 8, but only has 7, it thinks it's 1 short an electron, so it's always looking for an extra. It is "Positive" in nature, seeking out the extra electron to make it "neutral". Now remember, no one has ever actually seen an electron, so everything we think we know about this is theory, and not law. While we can use theories for educational purposes, we should always strive to question them until they are proven by facts to be true or false. Some believe that when "P" type doping is mixed into the Silicon crystal lattice, they form "holes" in the lattice where a Silicon electron has nothing to bond to. Holes can conduct current, and that the hole accepts an electron from a neighboring atom, moving the hole over a space. Thus P-type Silicon is a good conductor.  Now let's think about this. If indeed there are holes, the hole itself would have no electrical charge, positive or negative, and so would not "attract" electrons as one would think a proton would. The lack of an electron does not necessarilly demand a positive entity, although it would be less negative than an electron, so would be percieved to be more positive than the electron. Indeed, each individual atom in the latice has an equal number of protons for electrons, and so in essense is the molecule as a whole is electrically neutral.
Now let's think about this. If indeed there are holes, the hole itself would have no electrical charge, positive or negative, and so would not "attract" electrons as one would think a proton would. The lack of an electron does not necessarilly demand a positive entity, although it would be less negative than an electron, so would be percieved to be more positive than the electron. Indeed, each individual atom in the latice has an equal number of protons for electrons, and so in essense is the molecule as a whole is electrically neutral.In essence, what truly happens is that the almost full outer ring lacks 1 electron from being in a "complete" state. Even though each atom has the same amount of electrons and protons, and is electrically neutral, it desires to have an exact number of electrons in its valance ring to fill it out, and if it has less, goes looking for another one... and so in this manner, it seeks to fill or complete its valance ring. In either case, "P-type" doping is created by adding an element that is shy 1 electron. Even the smallest amount of either P-Type or N-Type doping changes a Silicon crystal from an insulator into a possible conductor. But because it isn't a true conductor, it is called a semi-conductor. Each type by itself is interesting, but it is when the two are joined together that the magic begins! It is at the point of the P-N junction itself that things get interesting. Now on to the rest of the course... Click Here to Continue |
| (On The Following Indicator... PURPLE will indicate your current location) | ||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
| 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 46 | 47 | 47a | 48 | 49 | 50 |
| 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 |
| Otherwise - please click to visit an advertiser so they know you saw their ad! |
